Objectives:
1. define the electrophiles and nucleophiles.
2. Explain the mechanism of electrophilic addition in alkenes.
3. Explain the chemistry of alkenes as exemplified by the reaction with :
Hydrogen, hydrogen bromide, bromine in CCl4, bromine water, water and concentrated sulphuric acid.
Activity 1 :
Electrophile : An electron deficient species that can accept a pair of electrons. Examples : AlCl3, BF3, NO2+, H+
Nucleophile : An electron rich species that can donate a pair of electrons. Example : H2O, NH3, OH-
- The p bond in alkenes is electron rich and hence, alkenes are more reactive than alkanes.
- The electrophile attacks the electron rich centre at the C=C forming a carbonium ion (carbocation) as an intermediate.
- This carbonium ion then combines with a nucleophile to form a product. Hence, alkenes undergo electrophilic addition reactions.
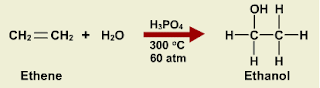
Activity 2:
1. Hydrogenation
CH2=CH2 + H2 à CH3CH3 catalysts : Ni, Pd or Pt
Uses : catalytic hydrogenation is used in industry to convert liquid vegetable oil to semi-solid fats in the
4. Markovnikov’s rule : When hydrogen halides are added to unsymmetric alkenes, the hydrogen atom is added to the carbon atom with a higher number of hydrogen atoms.
CH3 – CH=CH2 + HBr à CH3 – CH – CH2
Bromination of Alkene
Bromination of Alkenes in water
Q: Why is 2-bromoethanol the major product ? Water is in excess in the reaction.
Addition of sulphuric acid